CHICAGO — The Center for COVID Control, a locally based chain of COVID-19 testing pop-ups being investigated by a range of state and federal agencies, and its lab are being sued by the Minnesota Attorney General’s Office.
Minnesota Attorney General Keith Ellison announced the suit Wednesday. Ellison said the company and its lab, Doctors Clinical Lab, which has billed the federal government for more than $124 million since the start of the pandemic, gave people incorrect or falsified test results or didn’t deliver results at all.
The Center for COVID Control is based out of suburban Rolling Meadows and has 300 locations nationally, including many in Chicago.
Last week, Block Club revealed the chain’s lab has been cited at the highest level by a federal agency, and the companies are being investigated by various state agencies.
The Centers for Medicare & Medicaid Services, a federal agency that oversees the company’s go-to lab, has found “non-compliance” and “deficiencies” at the lab, according to a spokesperson. A December review from the agency cited the lab for “immediate jeopardy,” the most serious infraction, noting it had made mistakes that led to tens of thousands of PCR tests not being processed and workers weren’t following proper procedures for rapid tests, among other concerns.
Numerous Chicagoans told Block Club they received negative test results from Doctors Clinical Lab, only to get a positive elsewhere. Others never received results, or received them so late the test was effectively useless. Some people who didn’t even test at the sites were still sent results.
While that confusion has unfolded, the leaders of the company have bought a $1.36 million home and have posted online about buying cars worth hundreds of thousands of dollars — including saying they bought a Ferrari that cost $3.7 million. Akbar Syed, who represents himself as a leader of the company, posted that he was able to buy luxury cars thanks to “covid money.”
Doctors Clinical Laboratory has received more than $124 million from the federal government for tests and treatment for people who are supposed to be uninsured, according to public data.
The Illinois Department of Public Health and the Illinois Attorney General’s Office are investigating complaints with the company and its lab. Attorney general’s offices in multiple states told Block Club Chicago they’ve received complaints about the chain, as well.
The Better Business Bureau, a nonprofit that tracks consumer complaints about businesses, has given the Center for COVID Control an “F” rating and is investigating complaints about the chain.
Multiple customers tested at Center for COVID Control sites told Block Club they tried to call the company with questions and concerns — only to face customer service lines that were hours long with more than 100 people waiting, and no answer once you got to the front of the line.
The company has shut down its locations until Friday, with CEO Aleya Siyaj saying the staff is taking a temporary pause to work on training and complying with regulatory guidelines.
“Center for COVID Control is committed to serving our patients in the safest, most accurate and most compliant manner,” Siyaj said in a news release last week. “Regrettably, due to our rapid growth and the unprecedented recent demand for testing, we haven’t been able to meet all our commitments.
“We’ve made this difficult decision to temporarily pause all operations, until we are confident that all collection sites are meeting our high standards for quality.”
Read the full lawsuit:
Company ‘Deceived’ Minnesotans, Attorney General Says
The Minnesota attorney general’s office filed its lawsuit against the Center for COVID Control and Doctors Clinical Lab on Wednesday.
The lawsuit alleges the companies told consumers they’d get PCR test results in 24-72 hours and rapid test results the same day of their test. But many Minnesotans didn’t get test results at all or didn’t receive them within the time frames they’d been promised, representatives from the attorney general’s office said.
The companies also tested specimens that were older than 72 hours and could no longer provide accurate results, falsified dates on test results, told consumers their test results were “inconclusive” despite not knowing if their test had been processed or where it was, and sent negative test results to people who had never submitted a sample, according to the attorney general’s office.
Ellison said the company had “deceived” people.
“This false behavior is impacting real Minnesotans who are trying to stay safe from COVID,” Ellison said Wednesday.
The Minnesota agency is asking for injunctive relief, which would stop the companies from continuing their conduct, according to the office. It’s also asking for the company to face civil penalties of up to $25,000 per violation and to pay for the cost of the office investigating, as well as paying for litigation and attorney’s fees.
Two Minnesotans shared their stories of going to Center for COVID Control sites during a Wednesday news conference.
Hannah Puffer said she and her partner were tested more than a week ago. She was told she’d get rapid test results within 20 minutes, but she has yet to get results, despite calling the company multiple times and going to the site to ask for information, she said.
Edward Hugener said he and his 12-year-old daughter went to a site, hoping to get a rapid test site. But the site was “absolutely disorganized, disjointed,” with no social distancing or signs to wear masks, so they left, he said.
“I’ve never seen an organization run so poorly in terms of a process so concerning as COVID,” Hugener said.
Despite not having taken or submitted a test, Doctors Clinical Laboratory sent Hugener a negative test result that night, he said.
The next morning, the lab sent Hugener an email saying his daughter had taken a test that morning and was negative — but she was in school and couldn’t have tested, he said.
Hugener said the site was an “absolute disaster” and he’s happy the Minnesota attorney general’s office is suing.
The Minnesota agency began getting complaints about the company two weeks ago, said Jason Pleggenkuhle, Minnesota’s assistant attorney general. Several former employees of the company have come forward to work with investigators, as well, Pleggenkuhle said.
Those employees said the company’s headquarters in Rolling Meadows didn’t expand even as the company opened up more testing sites and took in more samples, according to the attorney general’s office.
Former employees said workers kept untested samples in garbage bags, unrefrigerated, piled around the office for days at a time, according to the attorney general’s office. They were told to falsify the dates they’d gotten tests and to lie to people who called in, telling them their test results were “inconclusive” or “negative” when their test had not actually been processed, according to the agency.
Former employees also said, in the “vast majority of” cases, they tried to get reimbursed from the federal government for tests, even in cases where people had provided information for their private insurance, Pleggenkuhle said.
That’s “very troubling,” Pleggenkuhle said.
The agency’s staffers would not comment on if they’re conducting a criminal investigation into the company’s billing practices.
“Here’s what I will tell you: We are investigating all avenues for accountability,” Ellison said.
From Axe-Throwing Lounges To COVID Tests
The Center for COVID Control is a management company to Doctors Clinical Laboratory. It provides tests and testing supplies, software, personal protective equipment and marketing services — online and printed — to testing sites, said a person who was formerly associated with the Center for COVID Control. Some of the sites are owned independently but operate in partnership with the chain under its name and with its guidance.
Doctors Clinical Lab is the lab that processes many of the chain’s tests and sends the results to patients.
The business and the lab are run by Siyaj and Syed, a suburban Chicago couple who, before focusing on COVID-19 testing in 2021, ran axe-throwing lounges. Syed also created wedding videos.
Siyaj lists herself as the CEO of the Center for COVID Control on LinkedIn, and Syed referred to himself as the “founding father” of the business on his Facebook until recently. He also posted videos trying to recruit people to the business on Facebook; in one, he recorded himself speaking to an employee and asking the man to say what he makes.
The man said he has been with the company for four months and makes $1.45 million.
Syed appeared to remove that title and videos after being contacted by reporters.
Siyaj bought a $1.36 million home in November, USAToday first reported. The nearly 7,000-square-foot home has crystal chandeliers, a two-story living room, a library with a fireplace, a “master wing” with a bedroom and a 4-acre yard, according to a listing.
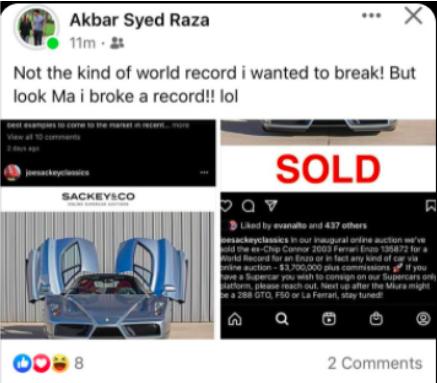
In Facebook posts, Syed wrote in December he had bought a rare Ferrari. An online listing said the car sold at auction for $3.7 million, not including commission.
Syed has also made references to the business on his TikTok, saying he’s been able to buy multiple luxury sports cars due to his work with COVID-19 testing, and writing that he owns dozens of testing sites and a lab.
In a post where Syed is shown bidding $400,000 for a Lamborghini at a car auction, someone asked him what he does for a living.
“My axe throwing lounges were forced shut by the gov due to covid,” Syed wrote on Aug. 17. “So I opened up a covid testing site than bought the lab and now i have 65 sites.”
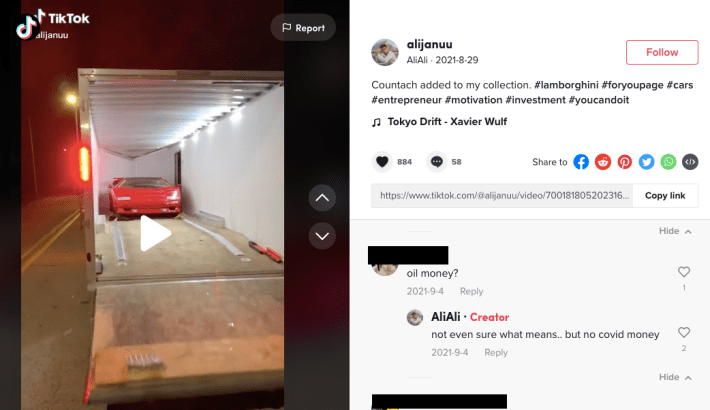
In an Aug. 29 video where Syed talks about buying a Countach, a luxury sports car, someone asked, “Oil money?” Syed replied, “Not even sure what means.. but no covid money.”
In another post, someone asked Syed how could he afford “all those cars.” “Covid testing,” Syed replied. “Rapid and pcr both.”
Syed’s nephew also posted videos on YouTube showing Syed “unveiling” a 2018 Ford GT, and other videos show the car in Syed’s driverway, USAToday reported. The same car sold for $985,000 in early December.
And in an exchange Dec. 20-21, someone criticized Syed’s business because they’d “been waiting for 2 weeks” for PCR results, he wrote.
“Give us another shot,” Syed wrote. “We are ready for the surge now.”
Syed’s TikTok account was taken down after reporters contacted him.
 Credit: Colin Boyle/Block Club Chicago
Credit: Colin Boyle/Block Club ChicagoFrustration Across The Nation
People going to Center for COVID Control sites across the United States have raised questions about numerous issues: dirty sites, long lines, crowded rooms, workers not wearing masks or gloves, workers trying to charge for tests that should be free or telling people to put down that they don’t have insurance when they do, among other problems.
But chief among many people’s concerns has been getting their results and ensuring they are accurate.
Center for COVID Control sites test thousands of people per day, and many have gotten results as expected. But Block Club spoke to people tested at various sites who said they never received results, experienced long wait times — sometimes weeks — before getting results or got back results that didn’t make sense to them.
Robert McNees, of Rogers Park, stopped by a Center For COVID Control site with his family Dec. 22. But the facility was crowded and “chaotic,” and the family wasn’t given instructions for doing the tests, McNees previously said. They opted not to take the tests or turn them in — but about five hours later, every member of the family was emailed a negative result from the company, he said.
Kristen Wylie, of Kalamazoo, Michigan, said she and her partner tested five times over a two-week period at one of the chain’s sites in her area. Her partner tested positive through a rapid test at the site on Dec. 20, but they both got negatives when they tested at the site in the days after that. They then got a PCR test at an unrelated pharmacy and both came back positive.
Trevin Cox, of Logan Square, stopped by a Center for COVID Control testing site Dec. 23 after being exposed. The site’s rapid test gave him a negative result, as did a rapid and PCR result at a pop-up under another chain. But Cox lost his sense of smell, had a fever and had other symptoms. An at-home test came back positive.
 Credit: Colin Boyle/Block Club Chicago
Credit: Colin Boyle/Block Club ChicagoJacob Bennett, of Lakeview, was tested Dec. 21 and still hasn’t received his rapid test results.
“It’s not useful if they don’t give you the results that were promised,” Bennett said. “I don’t have any reason to think that the actual testing is problematic — but not getting a result defeats the entire purpose.”
A Denver woman, who asked that her name not be used, took rapid and PCR tests Oct. 18 at a Center for COVID Control site in Colorado; her rapid result was positive. But her company required a PCR result, which the site hadn’t sent her. She called in, waiting about an hour and a half to speak to someone with the Center for COVID Control — and the worker she eventually reached told her, essentially, “We don’t know,” she said.
About a week after taking the test, the woman got her result emailed to her — and the PCR results said she was negative, she said. A PCR test she took at a state-run facility showed she was positive.
“Out of the three [tests] I took that week, theirs was the only one I was told ‘negative’ — and the one I waited the longest for,” she said. At another point, she said, “Which is scary because if you get that negative test and you haven’t received others, you’re probably going back out into the population like everything is OK.
“While that negative didn’t mean a whole lot to me, I was angry for the sake of others who may have been getting it and therefore spreading it. … You’re a huge role in people knowing that they’re positive and not spreading it. So if you’re giving out wrong results or fake results … that’s a huge issue.”
HELP US REPORT: Have you been tested at a COVID-19 pop-up? Click here to tell Block Club about your experience.
The chain has also leaned heavily on rapid nasal swab tests, even as studies have shown rapid antigen tests are less sensitive than PCR tests, producing more false negatives when someone has the virus.
Another concern for many: People who were emailed a negative test result were provided with a PDF that contained a QR code. Scanning the QR code took the viewer to a Doctors Clinical Lab website that tells anyone who looks at it they are negative — even people who never tested at a Center for COVID Control site.
The website was not coded in a way where its result would change, and it was not customized to show the results of individual patients. The only element that would change on the website was its timestamp. It was publicly available to all.
The website also contained a QR code that, if scanned, would take the viewer to a Google search of the word “negative.”
“That’s shocking. What the heck?” Wylie said after learning about the QR code. “Is this a scam? I’m shocked.”
The website was updated after reporters contacted the Center for COVID Control and Doctors Clinical Lab. It now says it cannot show the results of an individual due to HIPAA laws.
People who were sent a positive test result were also sent a QR code; that one went to the Google search for “positive.”

Investigations
The Center for COVID Control and its partner lab, Doctors Clinical Lab, are facing several investigations among federal and state authorities.
The company is “responding to queries” from health and regulatory agencies, and it will take its week of closure to ensure it’s complying with regulatory guidelines, according to a news release from last week.
The Centers for Medicare and Medicaid Services, which regulate and certify labs, have cited the Rolling Meadows-based lab for “immediate jeopardy”-level deficiencies — the highest level — in three areas: general laboratory systems, analytic systems and laboratories performing high complexity testing.
The deficiencies were found in various labs, including the main laboratory, on various days in November and December. The report was issued Dec. 8.
The Centers for Medicare and Medicaid Services “has a record of complaint surveys being performed at Doctors Clinical Laboratory,” an agency spokesperson said. The agency “identified non-compliance and is waiting for a response from the laboratory to the deficiencies cited.”
Inspectors from various states highlighted a number of issues in the report.
They said Doctors Clinical Lab didn’t have enough personnel to test all the PCR samples it got, and it did not have enough freezer space to store them, which resulted in more than 41,000 samples being unusable during an 11-day period in November.
Inspectors also found the lab wasn’t do quality control testing on the machine used to test samples, and workers at testing sites weren’t properly conducting rapid tests, among other issues.
 Credit: Colin Boyle/Block Club Chicago
Credit: Colin Boyle/Block Club ChicagoThe Center for COVID Control and Doctors Clinical Lab have also faced scrutiny from other agencies.
The Illinois Attorney General’s Office has received about 10 complaints about the Center for COVID Control and has opened an investigation, a spokesperson said. People who want to file a complaint can do so online.
Multiple attorney general’s offices in other states told Block Club they have received complaints about Center for COVID Control sites.
A spokesperson for the Illinois Department of Public Health said the agency is investigating complaints against the lab.
The Better Business Bureau, a non-government agency, is also looking into complaints about the business, said Steve Bernas, president of the agency’s Chicago division. The organization has given the Center for COVID Control an “F” rating, its lowest.
The organization has seen 11 complaints about the business filed since Dec. 20, Bernas said. The complaints have alleged they didn’t get test results, the test results were inconclusive or they paid for expedited testing results, but did not get results in the time that was promised, Bernas said.
 Credit: Colin Boyle/Block Club Chicago
Credit: Colin Boyle/Block Club ChicagoExperts have also raised questions about the company’s billing practices.
Doctors Clinical Lab, the lab Center for COVID Control uses to process tests, makes money by billing patients’ insurance companies or seeking reimbursement from the federal government for testing. Insurance statements reviewed by Block Club show the lab has, in multiple instances, billed insurance companies $325 for a PCR test, $50 for a rapid test, $50 for collecting a person’s sample and $80 for a “supplemental fee.”
In turn, the testing sites are paid for providing samples to the lab to be processed, said a person formerly associated with the Center for COVID Control.
In a January video talking to testing site operators, Syed said the Center for COVID Control will no longer provide them with PCR tests, but it will continue supplying them with rapid tests at a cost of $5 per test. The companies will keep making money for the rapid tests they collect, he said.
“You guys will continue making the $28.50 you’re making for the rapid test,” Syed said in the video.
Any time there is money flowing between a provider to any kind of patient, it raises concerns about the United States’ anti-kickback statute, said Jeb White, CEO of Taxpayers Against Fraud, a nonprofit dedicated to fighting fraud.
The statute prohibits organizations from receiving money in exchange for things like referring patients or patronage to a lab.
“At the very least, it is worth a heightened level of scrutiny to see if there is any quid pro quo playing out here,” White said.
Customers have also reported being told to not put down their insurance information even if they have insurance. In those cases, the federal government likely ends up paying for those tests.
Public data shows the federal government has reimbursed Doctors Clinical Laboratory more than $124 million for COVID-19 tests and “treatments.”
If workers are telling people not to put down insurance information, those charges are being passed to the government “needlessly” and creating a “harm” to the government, White said.
READ MORE
• Be Cautious Around COVID-19 Testing Pop-Ups, Illinois Attorney General Warns
Block Club Chicago’s coronavirus coverage is free for all readers.
Listen to “It’s All Good: A Block Club Chicago Podcast” here:
