In Summary: A scheduling dispute related to a Freedom of Information Act (FOIA) request for more than than 329,000 pages of COVID-19 vaccine data led to misleading social media posts in November 2021. The U.S. Food and Drug Administration (FDA) proposed a schedule to process and release 500 pages every month, arguing that this is the standard rate to process FOIA requests as “reviewing and redacting records for exempt information is a time-consuming process.” The FDA would start releasing this data immediately, but the full set of pages would not be processed until 2076. The FDA argued that the amount of time required to fulfill this request is due to the broad FOIA request that involves hundreds of thousands of pages.
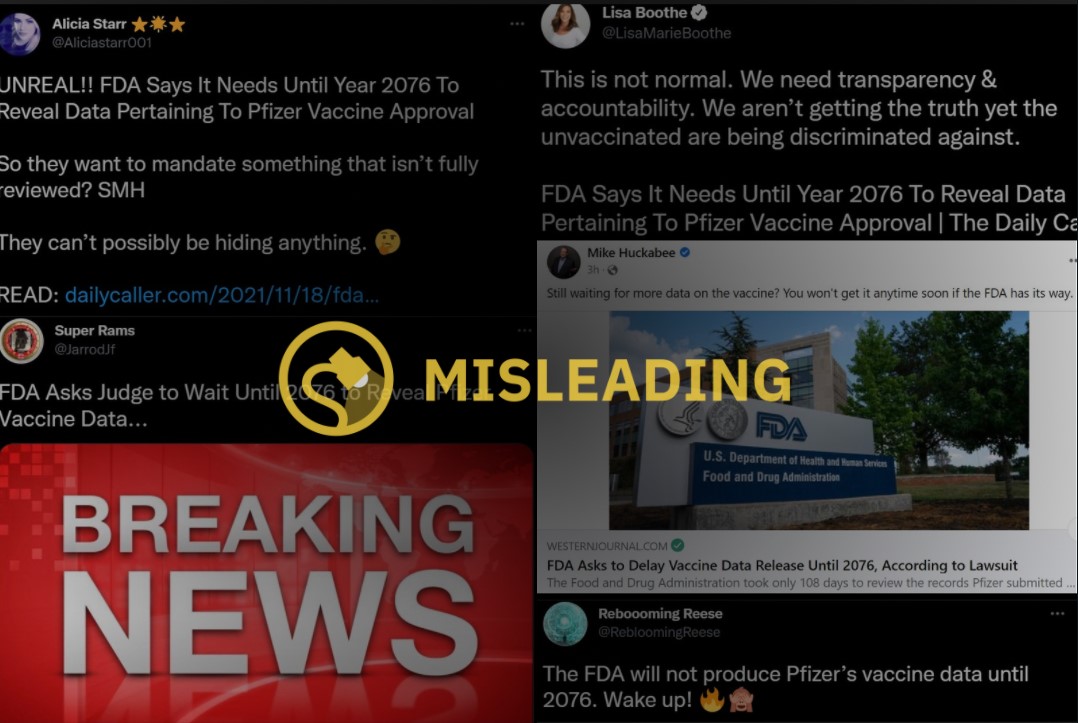
In November 2021, a rumor started circulating on social media that the U.S. Food and Drug Administration (FDA) was attempting to “hide” data related to the COVID-19 vaccine and that they had requested to delay the release of pertinent information until 2076.
The FDA did not request a delay in the release of its COVID-19 data until 2076. The FDA responded to a Freedom of Information Act (FOIA) request for more than 300,000 pages of data related to the licensure of Pfizer’s COVID-19 vaccine by proposing a processing schedule that would see the release of 500 pages every month. While the FDA argues that this is a rather standard processing schedule, if adhered to it would take the FDA more than 50 years, or until 2076, to completely fulfill.
An FOIA Request
This claim stems from an FOIA request filed by Public Health and Medical Professionals for Transparency for the data the FDA relied upon to license Pfizer’s COVID-19 vaccine. While the government is required to respond to these requests, the amount of time it takes to fulfill these requests varies depending on the number of documents requested, the backlog at the responding agency, and the complexity of the involved documents. The U.S. Department of Justice wrote:
Agencies typically process requests in the order of receipt. The time it takes to respond to a request will vary depending on the complexity of the request and any backlog of requests already pending at the agency. A simple request can be processed faster by the agency than one that is complex. Simple requests are typically more targeted and seek fewer pages of records. Complex requests typically seek a high volume of material or require additional steps to process such as the need to search for records in multiple locations. The agency’s FOIA Requester Service Center is available to assist you with any question about the status of your request and any steps you can take to receive a quicker response.
On November 15, 2021, the FDA and Public Health and Medical Professionals for Transparency presented a joint report to the U.S. District Court for the Northern District of Texas and are now waiting for the court to decide on a schedule for the release of these documents.
The Plaintiff’s Argument: 108 Days
The Public Health and Medical Professionals for Transparency (plaintiff) argued that the FDA should release these documents no later than March 3, 2022. The plaintiff chose this date as it gives the FDA 108 days, the same amount of time it took the agency to review the data to license Pfizer’s COVID-19 vaccine.
The plaintiff wrote:
Plaintiff seeks the records submitted to the FDA by Pfizer to license its COVID-19 vaccine (the “FOIA request”) and requests an order requiring the FDA to produce all documents responsive to its FOIA request no later than March 3, 2022. This 108-day period is the same amount of time it took the FDA to review the responsive documents for the far more intricate task of licensing Pfizer’s Covid-19 vaccine (the “Pfizer vaccine”).
[…]
It took the FDA precisely 108 days from when Pfizer started producing the records for licensure on May 7, 2021,14 to when the product was licensed on August 23, 2021. We assume, as the FDA has stated, that it conducted an intense, robust, thorough and complete review and analysis of those documents in order to assure that the Pfizer vaccine was safe and effective for licensure. The FDA now has an equally important task of making those documents available to the Plaintiff in this case and the public at large in at least the same timeframe.
The plaintiff went on to argue that the release of these documents is of the upmost importance as the “ability of a majority of Americans to participate in civil society, and even exercise basic liberty rights, are now contingent on receiving this product.”
The ability of a majority of Americans to participate in civil society, and even exercise basic liberty rights, are now contingent on receiving this product. For example, the White House’s recent Covid-19 Action Plan and executive orders have made receipt of this product a condition of employment for more than 6 million federal workers and contractors, 22 million healthcare professionals, 84 million private sector employees, and the enlisted and reserve members of our armed forces. There are few whose livelihood, education, service, and participation in civil society are not contingent on a government requirement to receive this product. On this basis alone, basic liberty and government transparency demand that the documents and data submitted by Pfizer to license this product be made available to Plaintiff and the public forthwith, precisely as contemplated by federal regulations.
The FDA’s Argument: 500 Pages per Month
The FDA argued in the joint report that the plaintiff’s request involves more than 329,000 pages and that reviewing and redacting these records will be a time-consuming process. The FDA proposed a schedule to process and release 500 pages a month, a rate that they argued is consistent with FOIA request processing schedules.
The FDA wrote:
Reviewing and redacting records for exempt information is a time-consuming process that often requires government information specialists to review each page line-by-line. When a party requests a large amount of records, like Plaintiff did here, courts typically set a schedule whereby the processing and production of the non-exempt portions of records is made on a rolling basis.
[…]
FDA has assessed that there are more than 329,000 pages potentially responsive to Plaintiff’s FOIA request. […] FDA proposes to work through the list of documents that Plaintiff requested FDA prioritize for production in order of priority and process and release the non-exempt portions of those records to Plaintiff on a rolling basis. FDA proposes to process and produce the non-exempt portions of responsive records at a rate of 500 pages per month. This rate is consistent with processing schedules entered by courts across the country in FOIA cases.
The FDA also addressed the plaintiff’s request to process this FOIA request in the same time the agency reviewed the data before licensing Pfizer’s COVID-19 vaccine, calling this a “specious argument” as these two review processes are not comparable.
The Court should flatly reject Plaintiff’s specious argument that because the scientists reviewing Pfizer’s Biologics License Application could do so on an expedited timeframe, the government information specialists should be able to do so in the same period of time. As should be apparent, the review conducted by FDA scientists when considering to approve a product is entirely different from the review conducted by FDA government information specialists when considering whether FDA must keep certain information confidential. Moreover, FDA’s FOIA office does not have nearly the same level of personnel or resources dedicated to process FOIA requests as FDA has marshaled to review license applications for live-saving products in the middle of a pandemic.
It should also be noted that while many on social media claimed that the FDA wanted to “delay” the release of data until 2076, the agency’s proposed schedule would see data start to get released almost immediately. While it would take years for the entirety of this FOIA request to be fulfilled, the FDA argued that this is due to the plaintiff’s broad request:
Although Plaintiff takes issue with the amount of time it will take to process 329,000 pages at a rate of 500 pages per month, such a result is due to its own broad FOIA request. Courts do not waiver from the standard 500 page per month processing rate even when a FOIA request would take years to process … FDA has invited Plaintiff to narrow its request by specifying records it no longer wants FDA to process and release, and Plaintiff has declined to do so. If Plaintiff decides to request fewer records, then FDA will be able to complete its processing at an earlier date.
When Will This Data Be Released?
While the FDA argued that processing 500 pages per month is standard for an FOIA request, government agencies have been tasked with processing documents at much faster rates when dealing with particularly significant or time-sensitive issues. In 2019, for example, U.S. District Judge Paul Englemayer ordered the U.S. Departments of State and Defense to produce 5,000 pages per month to fulfill an FOIA request related to the murder of journalist Jamal Khashoggi:
Federal agencies must produce thousands of pages monthly of records pertaining to the killing of Saudi dissident Jamal Khashoggi because learning about his disappearance as quickly as possible is of “paramount importance,” a judge said Tuesday.
Representatives of the U.S. Department of State and Department of Defense had told U.S. District Judge Paul A. Engelmayer that producing 5,000 pages monthly makes it impossible to respond in a timely fashion to other Freedom of Information Act requests.
Engelmayer ordered the agencies to get it done anyway, saying the disappearance of the Washington Post columnist and Saudi national was of “considerable public importance.”
According to Reuters, this scheduling dispute will likely be settled next month: “U.S. District Judge Mark Pittman “has set a scheduling conference for December 14 in Fort Worth to consider the timeline for processing the documents.”
Sources:
Act (FOIA), Freedom of Information. FOIA.Gov (Freedom of Information Act) Frequently Asked Questions (FAQ). https://www.foia.gov/faq.html. Accessed 19 Nov. 2021.
Gonzales, Richard. “Federal Judge Orders Release Of Khashoggi Records By U.S. Government.” NPR, 6 Aug. 2019. NPR, https://www.npr.org/2019/08/06/748877181/federal-judge-orders-release-of-khashoggi-records-by-u-s-government.
Siri, Aaron. “FDA Asks Federal Judge to Grant It Until the Year 2076 to Fully Release Pfizer’s COVID-19 Vaccine Data.” Injecting Freedom, 17 Nov. 2021, https://aaronsiri.substack.com/p/fda-asks-federal-judge-to-grant-it.
“Wait What? FDA Wants 55 Years to Process FOIA Request over Vaccine Data.” Reuters, 18 Nov. 2021, https://www.reuters.com/legal/government/wait-what-fda-wants-55-years-process-foia-request-over-vaccine-data-2021-11-18/.